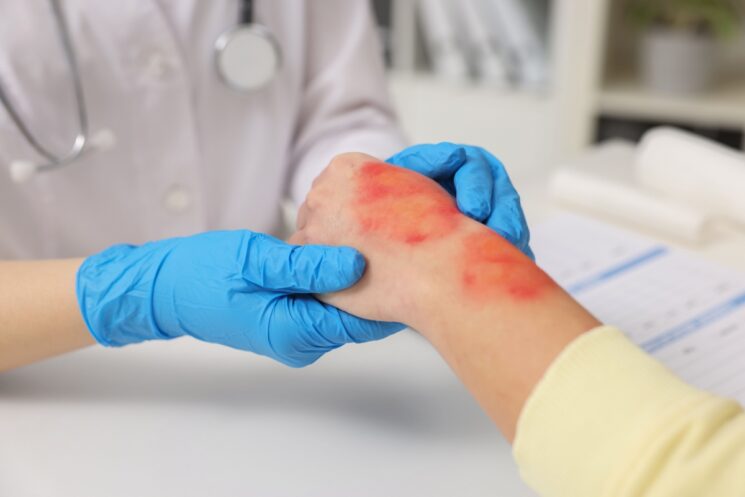
Amniotic grafts have become a fundamental part of regenerative medicine, offering clinicians an advanced biologic solution for wounds that do not respond to standard treatments. Among the different types available today, amniotic membrane grafts are proving to be the most effective form because of their ability to deliver vital biological signals directly to the wound bed. As chronic wounds continue to rise due to conditions such as diabetes, venous insufficiency, and aging, amniotic membrane grafts are shaping a new standard of care centered on natural, accelerated healing.
The Science Behind Amniotic Grafts
Amniotic grafts are derived from donated placental tissue following full-term deliveries. These grafts contain a rich network of structural proteins, growth factors, and natural antimicrobial components. What makes them unique is their compatibility with the human body and their ability to encourage tissue regeneration without triggering an aggressive immune response. This biologic harmony allows them to effectively support healing in wounds that are stalled in chronic inflammatory phases. The amniotic membrane offers a natural extracellular matrix that encourages cells to migrate, divide, and rebuild damaged tissue while simultaneously helping to reduce inflammation and fibrosis.
Why Amniotic Membrane Grafts Stand Out
While amniotic grafts can come in various processed forms, the amniotic membrane version plays the most critical therapeutic role because it delivers both biologic activity and functional protection. Once applied to a wound, the membrane acts as a protective cover while also signaling the body’s cells to restart stalled healing processes. It boosts epithelialization, supports collagen development, and encourages angiogenesis, the formation of new blood vessels needed to sustain new tissue growth. This multi-layer regenerative response is why the adoption of amniotic membrane grafts continues to expand across wound care specialties.
Clinical Impact: Reducing Inflammation and Promoting Regeneration
One of the greatest challenges in wound care is breaking the cycle of chronic inflammation. Amniotic membrane grafts help regulate inflammatory enzymes, creating an environment where tissue can rebuild rather than degrade. As the inflammatory burden decreases, the wound can transition into later healing phases characterized by tissue restructuring and closure. Patients often experience faster healing and reduced pain once the membrane is applied. The risk of infection is also lowered due to the naturally antimicrobial environment that the amniotic membrane fosters. This ensures that wounds not only heal faster but with fewer complications, improving long-term outcomes and reducing the likelihood of recurrence.
Preserving Biologic Integrity for Better Outcomes
The success of amniotic membrane grafts lies in how closely they maintain their natural properties throughout the processing and sterilization phases. Modern technologies now allow tissue banks to retain the structural basement membrane and extracellular matrix architecture that are essential for wound adherence and cellular communication. Because these components remain intact, the graft becomes more than just a barrier—it acts as a living biologic interface that integrates seamlessly with the patient’s tissue. Whether delivered in dehydrated form for convenience or cryopreserved for maximum cellular activity, properly preserved amniotic membrane grafts provide durable and dependable clinical performance.
Broad and Expanding Uses Across Medical Specialties
Although these grafts were first introduced in ophthalmology to support corneal regeneration, their widespread healing potential has made them valuable across many specialties. In podiatry, they help manage difficult diabetic foot ulcers that often lead to amputation if untreated. Burn surgeons use them to protect partial-thickness burns and donor sites where new skin must regenerate quickly to prevent infection and scarring. Orthopedic and sports medicine specialists apply them to surgical incisions and soft-tissue injuries to accelerate recovery and reduce lengthy downtime. Even urogynecologic and reconstructive surgeons are adopting them to support healing in delicate tissue areas. This wide-ranging effectiveness continues to drive research and expansion into additional clinical disciplines.
A Superior Alternative to Traditional Skin Substitutes
Traditional skin substitutes often focus on covering a wound rather than healing it at the cellular level. Some products offer structural support yet lack the biological elements needed to stimulate tissue restoration. Amniotic membrane graft for wounds are different—they provide both structure and signaling. They contain growth factors that activate fibroblasts, keratinocytes, and endothelial cells involved in skin and soft-tissue regeneration. Their anti-scarring properties help reduce excessive collagen formation, resulting in healed skin that is more functional and flexible. Because immune rejection is minimal and patient discomfort decreases rapidly after application, amniotic membrane grafts deliver advantages that many synthetic or animal-derived substitutes cannot match.
The Future of Regenerative Wound Care
As the medical community continues to shift toward biologics and regenerative therapies, amniotic membrane grafts are positioned at the center of innovation. Ongoing research aims to further optimize their biological activity, improve affordability, and explore combination therapies involving stem cells or controlled-release growth factors. Healthcare systems are increasingly recognizing the cost-saving potential of accelerated healing, fewer amputations, and reduced hospitalizations. With the growing demand for advanced wound care solutions, amniotic membrane grafts represent the future direction of wound management—one that prioritizes functional recovery and long-term patient health rather than temporary relief.
Conclusion
Amniotic membrane grafts are transforming the role of amniotic grafts in healing by delivering true regenerative power directly to wound sites. Their natural biological structure, ability to rapidly reduce inflammation, low risk of complications, and wide applicability make them one of the most important advancements in wound care today. As clinical adoption increases and innovations continue to emerge, these grafts are expected to define a new standard in tissue repair—one that restores health, mobility, and quality of life for patients facing the challenges of chronic and acute wounds.